O’levels Chemistry Notes
Periodic Table
By Mahvish Nadeem
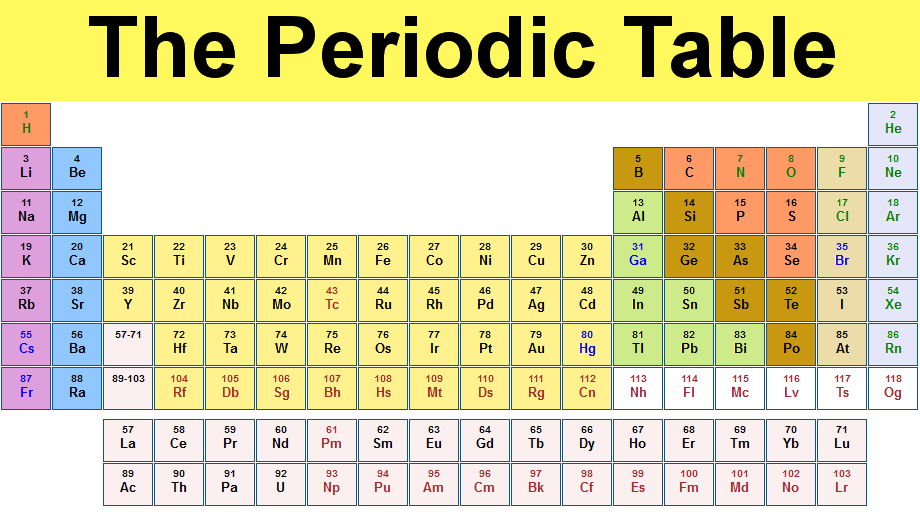
Features of the Periodic Table
1.The Periodic Table is a list of elements arranged in order of their increasing proton (atomic) numbers.
2. It consists of seven horizontal rows of elements called periods, numbered 1 to 7 from top to bottom.
There are also eight vertical columns of elements called groups, numbered from I to 0 or VIII.
Periodic Trends
Metallic and non-metallic characteristics
The staircase line in the Periodic Table dividers the elements into metals and non-metals.
Metals are grouped on the left-hand side of each period, non-metals and the right-hand side.
Elements located close to the staircase line (silicon, germanium) are called metalloids. Due to their positions, they have the properties of both a metal and a non-metal, and are used in the semiconductor industry which makes silicon chips for computers.
As a period is crossed from left to right, there is a decrease in metallic properties and an increase in non-metallic properties of the elements.
Across each period, oxides of elements change from basic to acidic, elements near the dividing line form amphoteric oxides.
Group & period numbers and electronic configuration
The period number indicates the number of electron shells.
The group number indicates the number of valence electrons. Elements in the same group have similar chemical properties (since they have similar electronic configurations).
Given the proton number, an element’s position in the Periodic Table and hence its properties can be deduced.
E.g. Sodium: 2.8.1
Since there are three shells, it is in period 3.
Since there is one valence electron, it is in group I.
Note: Hydrogen is not in any group.
Trends down a group
Proton number and relative atomic mass increases
Atoms become bigger (due to larger number of shells)
Properties of the elements become more metallic (has higher tendency to lose electrons/lose electrons more readily)
Ion formation and group number
Elements in Groups I, II and III
Are metals and lose electrons to form positive ions
The charge of the ion is the same as the group number
Elements in Groups IV and V
Less likely to form ions, they share electrons to form covalent bonds
Have a maximum oxidation state that is same as the group number (maximum oxidation state of P in PCl5, +5)
Elements in Groups VI and VIII
Non-metals and gain electrons to form negative ions
Elements in Group 0
Have stable electronic configurations
Unreactive and do not form compounds
Group I- Alkali Metals

Properties down Group I
Highly reactive, stored in oil to prevent them from reacting with air and water
One valence electron
React with cold water to form hydrogen and an alkali (the metal hydroxide)
Refer to TB/290 for reaction of alkali metal with water
Powerful reducing agents: they lose their single valence electron readily
Li ® Li+ + e-
Group VII- Halogens


A more reactive halogen will displace a less reactive halogen from its halide solution.
[halogen] [halide]
E.g. chlorine + sodium bromide ® sodium chloride + bromide
(colourless) (colourless) (colourless) (reddish-brown)
Chlorine acts as the reducing agent while the bromide ion acts as the reducing agent. Chlorine oxidises bromide ions to bromine and is itself reduced to chloride ions.
Group 0- Noble Gases

Periodic Table
By Mahvish Nadeem

Features of the Periodic Table
1.The Periodic Table is a list of elements arranged in order of their increasing proton (atomic) numbers.
2. It consists of seven horizontal rows of elements called periods, numbered 1 to 7 from top to bottom.
There are also eight vertical columns of elements called groups, numbered from I to 0 or VIII.
Periodic Trends
Metallic and non-metallic characteristics
The staircase line in the Periodic Table dividers the elements into metals and non-metals.
Metals are grouped on the left-hand side of each period, non-metals and the right-hand side.
Elements located close to the staircase line (silicon, germanium) are called metalloids. Due to their positions, they have the properties of both a metal and a non-metal, and are used in the semiconductor industry which makes silicon chips for computers.
As a period is crossed from left to right, there is a decrease in metallic properties and an increase in non-metallic properties of the elements.
Across each period, oxides of elements change from basic to acidic, elements near the dividing line form amphoteric oxides.
Group & period numbers and electronic configuration
The period number indicates the number of electron shells.
The group number indicates the number of valence electrons. Elements in the same group have similar chemical properties (since they have similar electronic configurations).
Given the proton number, an element’s position in the Periodic Table and hence its properties can be deduced.
E.g. Sodium: 2.8.1
Since there are three shells, it is in period 3.
Since there is one valence electron, it is in group I.
Note: Hydrogen is not in any group.
Trends down a group
Proton number and relative atomic mass increases
Atoms become bigger (due to larger number of shells)
Properties of the elements become more metallic (has higher tendency to lose electrons/lose electrons more readily)
Ion formation and group number
Elements in Groups I, II and III
Are metals and lose electrons to form positive ions
The charge of the ion is the same as the group number
Elements in Groups IV and V
Less likely to form ions, they share electrons to form covalent bonds
Have a maximum oxidation state that is same as the group number (maximum oxidation state of P in PCl5, +5)
Elements in Groups VI and VIII
Non-metals and gain electrons to form negative ions
Elements in Group 0
Have stable electronic configurations
Unreactive and do not form compounds
Group I- Alkali Metals
- The alkali metals are Lithium, Sodium, Potassium, Rubidium, Caesium, Francium.
- Physical Properties of Alkali Metals
- Soft, silvery solids
- Low mp and bp
- Low densities (Li, Na and K float on water)
- Compounds of alkali metals are soluble in water

Properties down Group I
- Densities increase
- Mp and bp decrease
- Reactivity increase
- Reducing power increase
- Chemical Properties of Alkali Metals
Highly reactive, stored in oil to prevent them from reacting with air and water
One valence electron
React with cold water to form hydrogen and an alkali (the metal hydroxide)
Refer to TB/290 for reaction of alkali metal with water
Powerful reducing agents: they lose their single valence electron readily
Li ® Li+ + e-
- Reactivity increases down the group: As we go down group I, the size of the atom increases (Na atom is bigger than Li atom due to extra shell). Since it is easier to lose the valence electron from bigger atoms, the reactivity increases down group I.
Group VII- Halogens
- Elements in group VII are called halogens and are non-metals. They are Fluorine, Chlorine, Bromine, Iodine and Astatine.

- Exist as diatomic covalent molecules: F2, Cl2, Br2, I2.
- Low mp and bp
- Coloured
- Properties down Group VII

- Mp and bp increase, change from gas to solid
- Colours of the halogens become darker
- Reactivity decreases down the group (Fl most reactive)
- Oxidising power decreases
- Chemical Properties of Halogens
- Reactive non-metals (7 valence electrons)
- React with most metals to form halides. Fl, Cl and Br ions are examples of halide ions.
- Powerful oxidising agents: halogen atom gain electrons to form halide ions during chemical reactions.
- Displacement reactions (redox)
A more reactive halogen will displace a less reactive halogen from its halide solution.
[halogen] [halide]
E.g. chlorine + sodium bromide ® sodium chloride + bromide
(colourless) (colourless) (colourless) (reddish-brown)
Chlorine acts as the reducing agent while the bromide ion acts as the reducing agent. Chlorine oxidises bromide ions to bromine and is itself reduced to chloride ions.
Group 0- Noble Gases

- The group 0 elements are called noble gases/ inert gases, and are the least reactive elements.
- Unreactive and stable: have noble gas structure hence do not react to form compounds
- Monatomic elements
- Low mp and bp that increase down the group
- Colourless gases at room temperature, insoluble in water

